What’s wrong with gene therapy?
For the last few years, every week brings a new headline with tantalizing breakthroughs in gene therapy. Each fawning science article promises that a genetic solution to aging, disease, and cancer is just around the corner.
Yet here I sit, growing older and unhealthier by the minute. Where’s my magical pill to cure all diseases? Why aren’t we curing cancer patients by giving them a shot of magical CRISPR-driven gene therapy and then sending them off to go play beach volleyball?
We hear about a lot of new discoveries in genetics, but they don’t seem to translate to clinical solutions. Where do they go wrong?
I’m often asked this question, so I thought I’d discuss some of the biggest reasons why we aren’t genetically engineered, super-strong, cancer-proof, healthy, nearly immortal beings.
The Hydra of Cancer
No, not the enemy Nazi-esque organization in Marvel’s Captain America comics and movies — although their catchphrase is apt. Whenever intrepid Captain America unmasked an agent of Hydra, they’d glare defiantly up at the hero and make their final declaration:
“Cut off one head, and two more shall take its place! Hail Hydra!”
That utterance is surprisingly similar to how cancer works — and why it proves so difficult for us to eradicate.
We’re used to thinking about disease in the framework of Robert Koch’s Germ Theory. Koch put forth four postulates, stating that every disease was caused by a specific microorganism. Introduce the microorganism to a susceptible host, and the host would get sick. Remove the microorganism, and the host would recover and return to health.
This approach worked great for a lot of diseases that have, in the past, ravaged humanity. For example, tuberculosis is caused by a specific bacterium, Mycobacterium tuberculosis. Introduce that particular species of bacteria to a vulnerable person, and they’ll come down with the disease. Eliminate the bacterium, and the disease goes away.
But although we treat cancer as a disease, it’s not. There are many different types of cancer — and I’m not talking just about physical location, like skin cancer, breast cancer, or lung cancer.
Normally, all the machinery inside a cell works, and that cell does what it’s supposed to do inside our body — store fat, or produce stomach acid, or break down nutrients. Cells accumulate mutations over time, random bits of damage to their DNA instructions, that eventually leads to errors in the cell’s machinery so it no longer works. In most cases, the cell detects that it can no longer function, and shuts itself down — a process called apoptosis. But in some cases, the cell doesn’t shut down, and begins growing uncontrollably as it tries to make sense of the mixed-up, error-filled signals from its own instructions.
This is cancer.
The problem? Just like any complex machine, there are lots of ways that a cell’s instructions can break. There are dozens of pathways, thousands of different possible mutation combinations that can lead to cancer. It’s not just one or two mutations — we’re talking about tens to hundreds of different places where the DNA instructions are no longer correct.

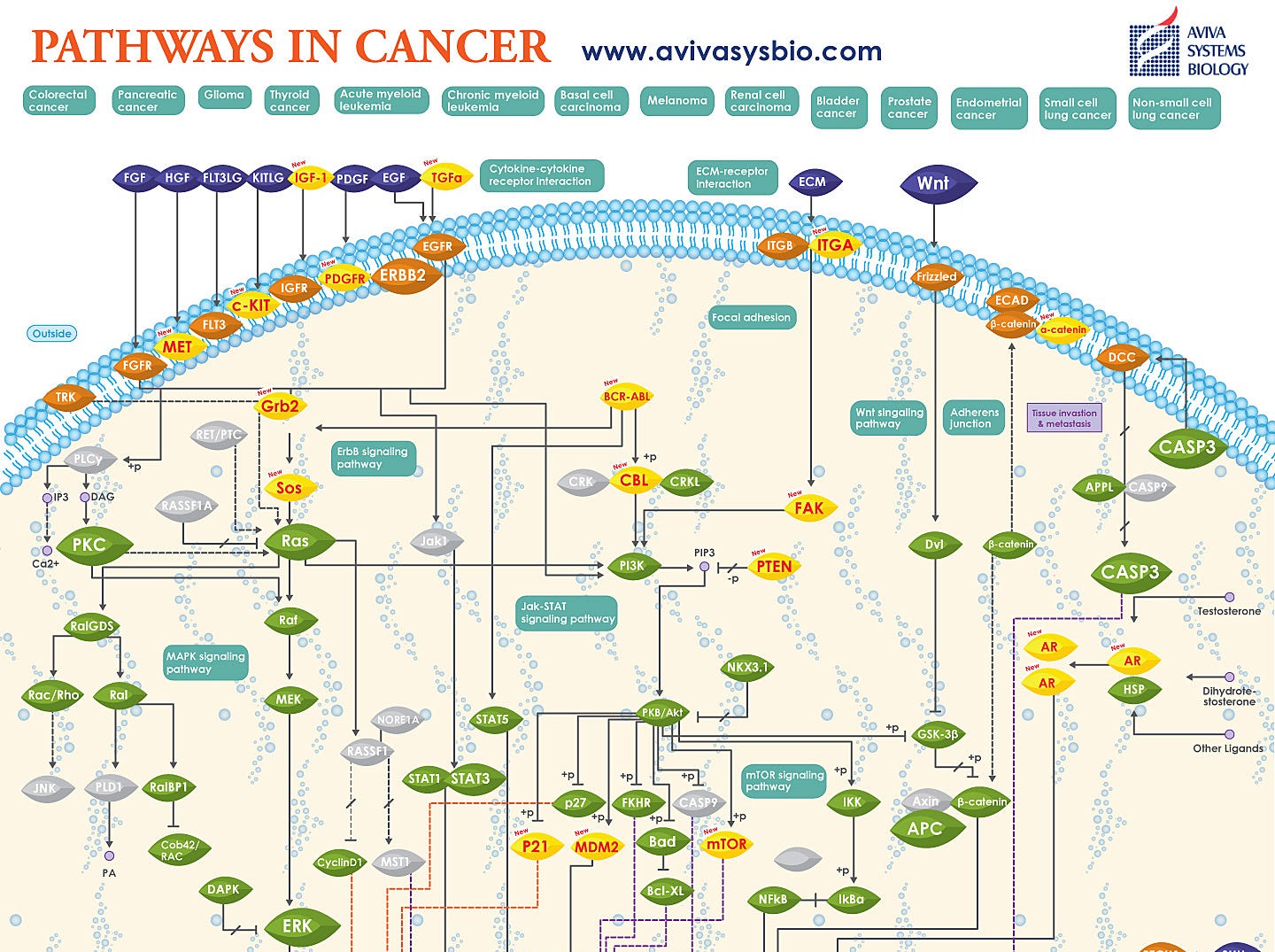
Gene therapy, as we know it, could fix one mutation at a time. But for cancer, we need to make many different changes, all at once. A good analogy would be if I handed you the entire Harry Potter series, translated into Portuguese, and told you to fix all the spelling mistakes.
Oh, and if you don’t finish within twenty-four hours, the patient dies.
Gene therapy has cancer in its sights — but each variant of cancer will require a customized approach. We’re starting to see solutions for very specific types of cancer, but we’re highly unlikely to have a generalized gene therapy approach for all cancers.
The Complexity of Traits
No, it’s not Darwin’s wildly anticipated best-selling follow-up to The Origin of Species. The complexity of traits is a huge challenge in genetics today, and one of the reasons why we geneticists hem and haw when asked if someone’s oddity is inherited or not.
Imagine if we took a baby and raised it in a caring, nurturing environment. That baby — let’s call him Albert — grows up with two caring, loving parents who encourage him and can afford for him to participate in extracurricular activities and get one-on-one tutoring. Albert studies hard, succeeds in school, and eventually goes on to make several vital discoveries in the field of physics.
But what if Albert grew up in a different environment? Same baby, but this time little Albert grows up with a single mother, siblings who compete for meager food and opportunities, and in an impoverished inner-city area with bad schools. He doesn’t get any one-on-one tutoring, and he has to work a part-time job to help support his siblings.
Do you think that, in both cases, the Alberts would be equally intelligent? Would they get the same score on IQ tests? Would both versions of Albert make those vital discoveries in physics?


The answer, as I’d imagine you’d guess, is no. Environment contributes a huge factor to certain traits, and the more complex of a trait, the more of the contribution may come from the environment. Intelligence is one of the most complex traits we can name, and it’s not something we can genetically predict or control.
There are stories today wondering if technologies like CRISPR will be used to make gene-edited super-intelligent babies. The answer, at least for the short term, is no. We don’t know which genes might improve intelligence — and even if we turn those genes on, the environment in which these “super-babies” are raised will have a far stronger influence on their intelligence than their genetics.
Intelligence is one of the most complex traits we can name, and it’s not something we can genetically predict or control.
Even traits that we’d consider to be fairly simple and largely inherited, like height, are surprisingly complex to unravel. In one recent study, more than a hundred and eighty different loci — specific points on the human genome — were correlated with height. Genetically building the next Yao Ming is not a matter of flipping a single switch; it may take hundreds of switches in the precisely correct configuration — and then, of course, the child needs the proper diet and exercise regimen to reach that height potential.
CRISPR is Just a Crappy Pair of Scissors


Whenever gene therapy is discussed in the news today, it’s almost always accompanied by a mention of the most popular gene-editing technology — CRISPR. CRISPR, or more specifically the CRISPR-Cas9 complex, is a method for making cuts in DNA at specific locations. It’s a molecular pair of scissors.
CRISPR is made of two components: the CRISPR guide RNA is a single strand of RNA (like half a DNA strand) that acts as the “guide”, telling the enzyme where to make a cut in the DNA. It’s attached to a Cas9 bacterial protein, which is what acts as the actual scissors to cut the DNA at the place where the guide CRISPR RNA matches up.
CRISPR isn’t the first pair of molecular scissors that we’ve discovered. Before CRISPR we had TALENs, and before TALENs we had zinc finger nucleases. Compared to these earlier methods, however, CRISPR is more accurate and less bulky, and because we can swap out that guide RNA to target whatever region of DNA we want, it’s very easy to customize.
However, CRISPR isn’t perfect. Despite all its advantages, it has a few problems.
First, it’s not totally accurate. Yes, it’s more accurate than the older forms of molecular scissors — but in the 3 billion base-pair human genome, there’s a lot of space for off-target effects. When you’re modifying a human genome, you don’t want to have any places where your modification “misses” and causes other effects. This could lead to cancer, spontaneous abortion of cells, or other unanticipated negative effects. There are attempts to improve the accuracy of CRISPR’s targeting, but they’re still in the early testing phases.
Second, that Cas9 protein is not something that our cells tolerate. The Cas9 protein comes from bacteria, and when our immune system sees a bacterial protein, it tends to react by launching an immune response against the perceived threat. This isn’t a huge issue if you’re editing a single-celled human embryo, but if you’re treating to treat a sick, adult patient, you don’t want to give them a treatment that will further trigger their already-stressed immune system.
CRISPR researchers are searching for improvements to fix both of these issues. There are promising methods for tackling both of these issues — modifying the RNA may improve its accuracy, and we’re building synthetic, stripped-down versions of the Cas9 protein that are less of an immune trigger without sacrificing their cutting function.
But we’ve got a ways to go. CRISPR is a huge improvement over previous technologies, but it’s not yet ready to be used for every modification.
Gene Therapy Must Stay on Target

With all these issues, is gene therapy a lost cause?
Definitely not. There are plenty of tempting, low-hanging fruit targets for gene therapy. Certain diseases can be caused by a single malfunctioning gene, can be detected early, and can potentially be fixed by a single snip to the individual’s genome. These diseases are also deadly enough to be worth the risks of gene therapy if they can be cured. If you know you’re going to get cystic fibrosis and likely die before your thirtieth birthday, would you risk cancer to cure it through a genetic intervention?
In the next few years, we’ll likely see gene therapy hit the clinical scene, used for treatments for these low-hanging fruit diseases, and also to target particularly malignant variants of cancer. But there’s a huge jump to get from curing specific, deadly diseases to adding enhancements to the human body.
As anyone who’s ever worked on complicated technology knows, there are lots of ways to break a process, but very few ways to make that process better. If you attack a car with a wrench, there are tons of ways to break the car. It’s very difficult, however, to make your car run more efficiently.
Gene therapy is similar. The human body is highly adapted and incredibly complex. We can figure out what’s broken when things go wrong, and we can maybe even fix those breaks. But it’s much, much more difficult to come up with a way for the human body to work more efficiently than it currently does.
Someday, further down the road, gene therapy may help us improve on what Nature has designed for us. But we first need to solve many issues — challenges with discovering a place to improve, figuring out what precisely to change, and then working out the engineering challenges in order to make those alterations.
For now, I’m not worried about genetically designed super-babies beating out my future kids for spots at an elite preschool.
